In-Vitro Diagnostic Medical Devices Regulation (EU) 2017/746
Transition periods and requirements

Since May 26, 2022, the IVDR has been officially in effect, replacing the IVDD. However, to ensure the transition is as smooth as possible, there are transition periods of varying lengths for products in different risk classes.
Furthermore, some IVDR requirements already apply to products that were certified under the IVDD before or during the transition period. Timo Brüggemann discusses these requirements and when notification to the notified body is necessary in this article.
EU Regulation 2017/746 on in vitro diagnostic medical devices (IVDR) replaced Directive 98/79/EC IVDD on May 26, 2022 . The IVDR requirements apply to all products placed on the market or put into service from May 26, 2022.
According to these requirements, a notified body must now be involved in the conformity assessment procedure for the majority of products. However, the number of notified bodies for conformity assessment under the IVDR is currently small. To ensure smooth transition and ensure the availability of products on the European market, the EU Commission is granting manufacturers extended transition periods (Regulation (EU) 2022/112). Furthermore, the sell-through period (Regulation (EU) 2023/607), which was in effect until the beginning of the year, has now also been lifted, although the respective shelf life of the products must be considered.
Extension of the transition periods
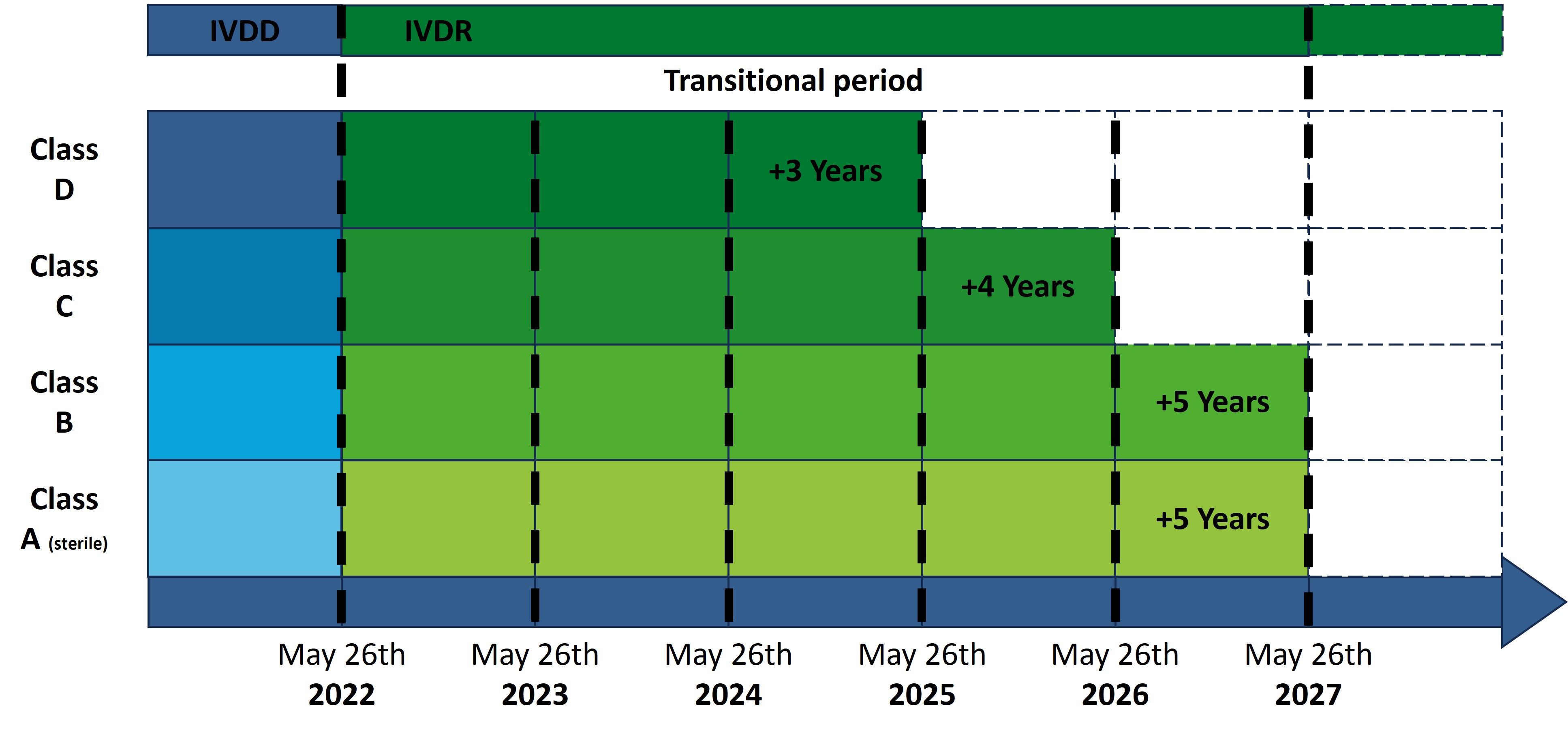
The extension of the transition periods is limited to existing products for which a declaration of conformity has already been issued before May 26, 2022. The extension depends on the risk class of the product and whether a notified body was already involved in the conformity assessment under the IVDD.
For products for which the involvement of a notified body was already required under the IVDD (List A+B and products for self-testing), the transition period is extended by one year, until May 26, 2025.
For products that require a notified body for the first time for conformity assessment according to the IVDR, the transition periods are now defined as follows:
- Class D products: May 26, 2025
- Class C products: May 26, 2026
- Sterile Class A products and Class B products: May 26, 2027
- For so-called in-house tests, the deadline is extended until May 26, 2028, provided that no comparable product is approved on the market.
Unsterile Class A products, as well as new products for which a declaration of conformity is issued after May 26, 2022, are excluded from the extended transition periods.
Obligations for Monitoring and Vigilance at IVDR Level
Notwithstanding this extension of deadlines, manufacturers must still fulfill their obligations regarding the monitoring of products on the market and vigilance, even if the products are still placed on the market under the IVDD.
The MDCG document 2022-8, published in May 2022, provides guidance on how to implement the requirements of the IVDR for existing products. The MDCG document divides existing products into 'Old' and 'Legacy' Devices; however, these terms are not mentioned in the IVDR and are only used in MDCG documents.
Depending on whether an existing product is an 'Old' or a 'Legacy' Device, different requirements apply until the specified deadlines.
‚Legacy‘ Devices
'Legacy' Devices are products that are placed on the market between May 26, 2022, and the aforementioned respective end of the transition period, provided that they do not undergo significant changes with respect to design and intended use. These can be products for which a certificate was already issued by a notified body under the IVDD before May 26, 2022, or products for which a declaration of conformity was issued before May 26, 2022, under the IVDD without the involvement of a notified body, and for which the involvement of a notified body is now required under the IVDR.
For 'Legacy' Devices, the IVDR requirements for post-market surveillance (PMS) and vigilance (Chapter VII, IVDR) also apply. A PMS system based on a PMS plan is mandatory, as well as a Post-Market Performance Follow-Up (PMPF), which is part of this PMS system. Regardless of the risk class, every Legacy Device requires a PMS report (Art. 80, IVDR); if it is a Class C or D product, a periodic safety update report (PSUR) can be prepared voluntarily instead (Art. 81, IVDR).
In addition, economic operators of 'Legacy' Devices have obligations that they must fulfill; this concerns manufacturers (Art. 11, IVDR), authorized representatives (Art. 11, IVDR), importers (Art. 13, IVDR), and distributors (Art. 14, IVDR). In principle, the registration of products (Art. 26, IVDR) and the registration of economic operators (Art. 28, IVDR) must also be compliant with the IVDR. Since EUDAMED is not yet fully functional, special transitional provisions apply, which are described in Articles 112 and 113(3) of the IVDR.
‚Old‘ Devices
Old' Devices include products that were placed on the market before May 26, 2022, and for which a valid certificate was issued under the IVDD before May 26, 2022, or the applicable national regulations before the entry into force of the IVDD. The IVDR does not apply per se to these products; however, manufacturers must also fulfill their obligation to monitor the products on the market. This means that after May 26, 2022, the articles on market surveillance (Art. 88 to 95, IVDR) also apply by the competent authorities. To further ensure that all reports and analyses of serious incidents and field safety corrective actions (FSCAs) are processed in the course of PMS, the articles on serious incidents and resulting measures (Art. 82 and 84, IVDR) also apply.
Applicable IVDR Requirements during the transition period for 'Legacy' and 'Old' devices with respect to their intended use.
| 'Legacy device' | 'Old device' |
|---|---|
| Obligations of Manufacturers, Authorized Representatives, Importers and Distributors (Articles 10, 11, 13 and 14, IVDR) | |
| Registration of products and economic operators (Articles 26 and 28, IVDR) in accordance with Articles 112 and 113(3) | |
| PMS system with PMS plan (Articles 78, 79, IVDR) | |
| PMS Report (Art. 80) OR PSUR (Art. 81, IVDR) | |
| Vigilance (Articles 82 to 87, IVDR) | Serious incidents and resulting corrective actions (Articles 82 and 84, IVDR) |
| Market surveillance (Articles 88 to 95, IVDR) | Market surveillance (Articles 88 to 95, IVDR) |
What if there are changes to the product during the transition period?
All these deadline extensions and the limited IVDR requirements for 'legacy' devices only apply if there have been no significant changes to the products with regard to intended purpose and design during the transition period.
If there are changes, it must be recorded whether they were significant. If the changes are not significant, the product can continue to be certified under IVDD during the transition period until the respective transition period expires.
If the changes are significant, the product can no longer remain on the market under the IVDD and must be recertified under IVDR requirements. The responsible notified bodies verify and document the changes in writing and decide on the continued validity in the course of their monitoring work.
The MDCG document 2022-6, also published in May, provides guidance on which changes are considered significant and which are not. In general, only certain changes to the intended purpose and design are considered significant.
Changes to the Intended Purpose
For example, an expansion of the intended purpose or other important changes (such as intended users or changes in the principle of operation) are considered a significant change to the intended purpose. Only limiting the intended purpose is considered a non-significant change. Thus, the intended purpose may be restricted but not expanded or modified.
Changes to the Design
Significant changes to the design are changes to the principle of operation or changes that lead to an impairment of the benefit-risk ratio, even if these do not change the principle of operation.
The umbrella term "design" also includes software, ingredients and materials, as well as sterilization.
If there are no significant changes to the design, it must nevertheless be checked whether there are changes to software, ingredients/materials or sterilization that constitute a significant change to the product. If all these checks reveal no significant changes, the product can continue to be regarded as an 'old' or 'legacy' device. If there is a significant change, the product must be recertified under the IVDR.
Conclusion
In summary, the transition periods have been extended for products of all classes, for different lengths of time depending on the risk class (excluding non-sterile Class A products). However, market surveillance and vigilance must already take place at IVDR level for 'old' and 'legacy' devices certified under the IVDD. If there are significant changes in the transition period with regard to the intended purpose or the design of the 'old' or 'legacy' devices, the product must be recertified under the IVDR.

