OntoPMS
The PMS strategy must be sustainable, it has to fulfill the requirements, but also to use the knowledge gained in the company. The key lies in a good definition of important data sources and their continuous monitoring.
Internal data sources, such as sales data and complaint statistics, are at hand. They generally formed the basis of the PMS processes under the Medical Device Directive. Additional internet-based data sources offer a higher range and depth of information regarding such questions as where the product is used, under which circumstances, what experience is gained, etc.? However, these data sources are less readily available.
We at novineon interlock the PMS with the power of IT. The OntoPMS system offers an automatic data search and saves resources without neglecting PMS obligations. An automated system that transparently and continuously captures critical data sources and evaluates them for relevance based on predefined PMS criteria meets the requirements set by the Article 83 of the MDR.
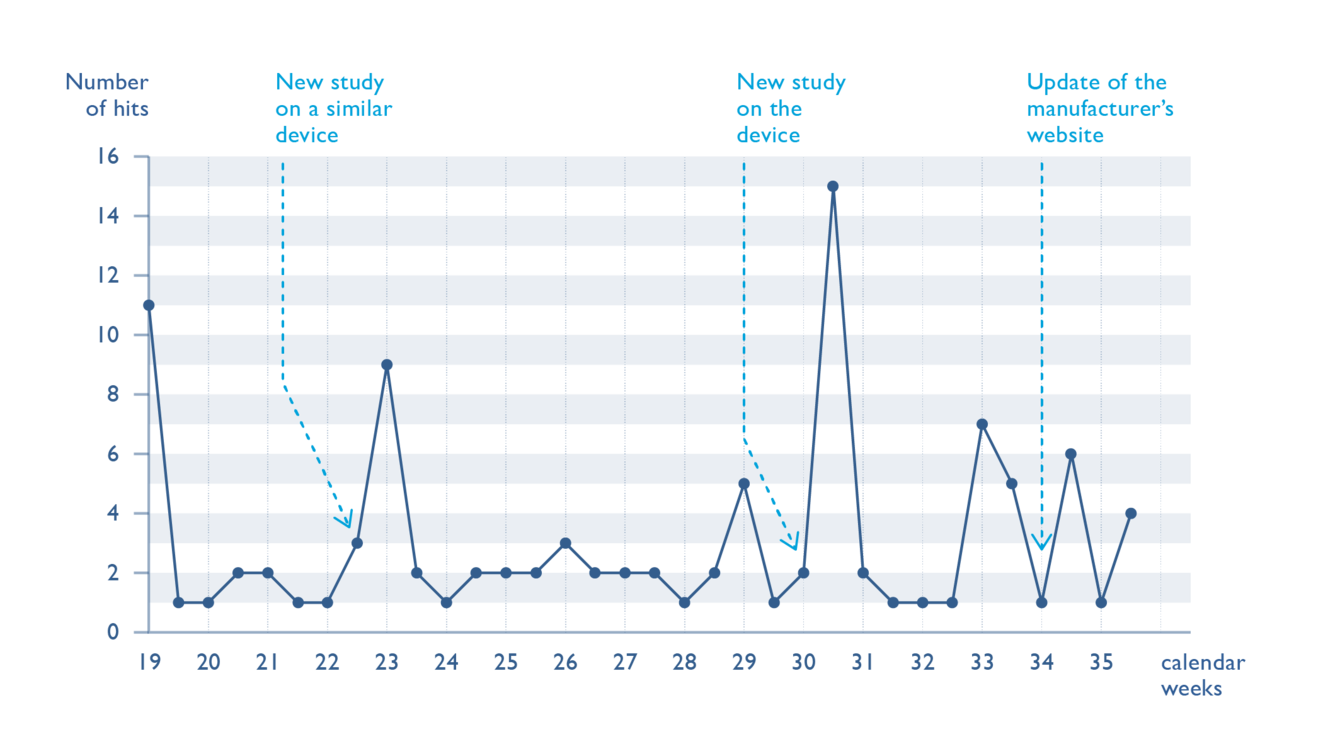
The intelligent recording of these data sources/URLs in a corresponding database enables a timely, central processing for the connected QM processes, such as clinical evaluation, risk management and trend reporting.
With regard to the entire PMS process of collecting, evaluating and reacting, the OntoPMS system focuses on continuous data collection and use. In particular, this covers data published on the internet.

